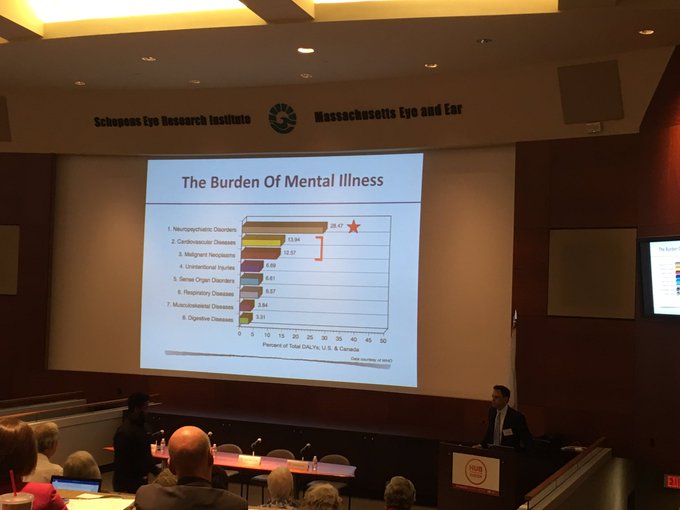
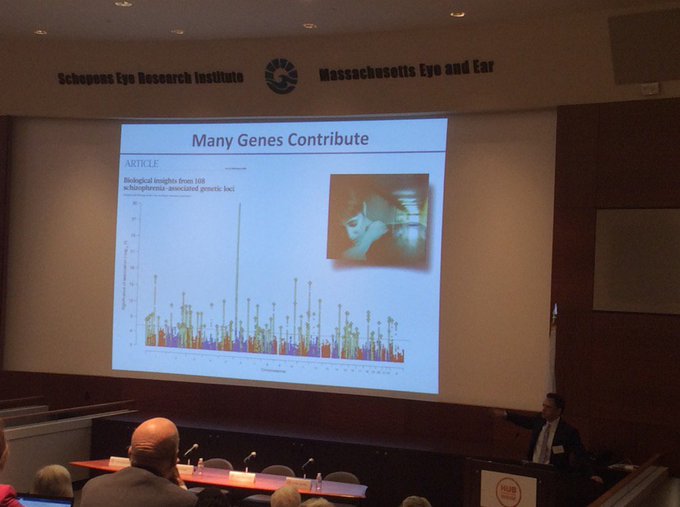
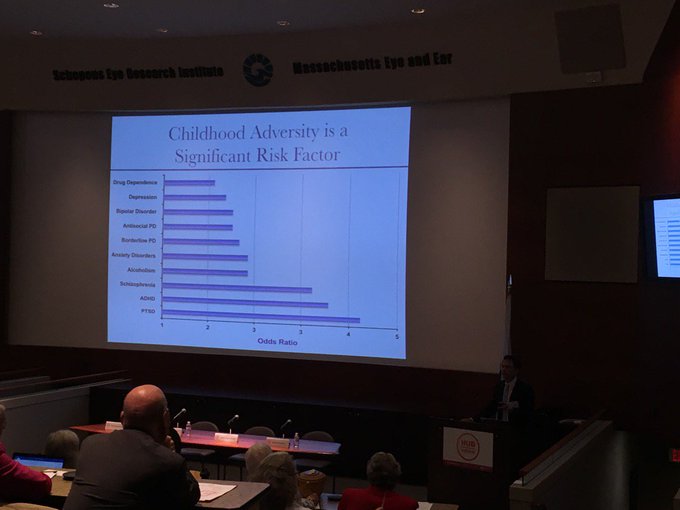
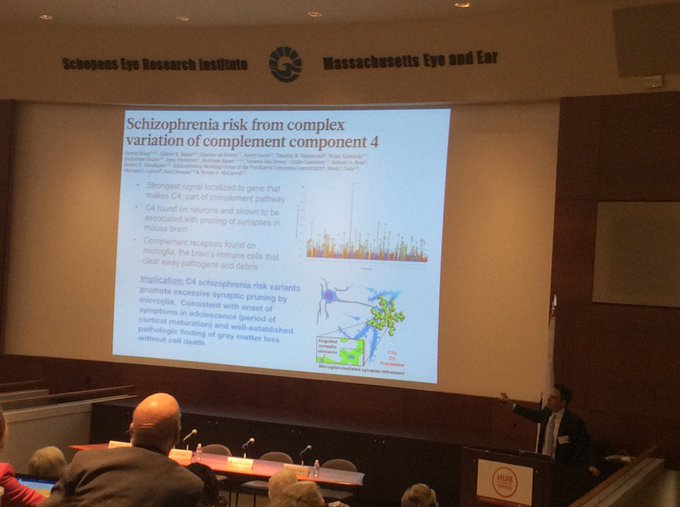
This
article was originally published by STAT (www.statnews.com) on December 21, 2016:
This
article was originally published by STAT (www.statnews.com) on December 21, 2016:
With the landmark 21st Century Cures Act now signed into law, Congress needs to turn its attention to another pressing health issue: the safety — or lack thereof — of medical devices.
Thousands of medical devices are on the market today, with a steady stream of new ones coming along. They include spinal and breast implants, pacemakers, TENS devices for pain, transvaginal mesh slings, tubal occlusion sterilization coils, dental amalgam, prosthetic joints, and more.
The Food and Drug Administration puts medical devices into three categories. Class I devices are simple and have little potential to cause harm, such as bandages. Class II devices are more complicated and present a greater risk or harm. These include motorized wheelchairs and simple implants such as radio frequency identification chips; they are often approved with special guidelines for control. Class III devices help support or sustain life or present a potentially significant risk of injury. Examples include pacemakers, artificial heart valves, and joint replacements.
For all the good that medical devices do, they can also cause harm. I know this because my health was almost irreparably harmed by the mercury-based dental amalgam used over my lifetime to fill cavities — it turns out that genetic susceptibility is a factor. I have friends, relatives, and colleagues who have endured cancers and autoimmune diseases, allergies, rashes, pain, memory loss, and incredible emotional strain from problems caused by various medical devices.
Click here to read the complete article. https://www.statnews.com/2016/12/21/medical-devices-safety-congress/